 SMEPAC test passed with HC dedusters and metal checks
SMEPAC test passed with HC dedusters and metal checks
Dedusters and Metal Checks Standardized Measurement of Equipment Particulate…
 Case study iSpray – External lubrication system
Case study iSpray – External lubrication system
The Challenge Punch sticking, hardness stability, and high ejection…
 New project industrial building
New project industrial building
Planning of the project June 2016 – November 2018…
 Pharma Technology thinks green
Pharma Technology thinks green
Pharma Technology headquarter is located in Nivelles in a…
 ACHEMA PULSE DIGITAL - June 15th & 16th
ACHEMA PULSE DIGITAL - June 15th & 16th
Join us on Achema Pulse from June 15th to…
 #PharmaTechnologyDoesNotStop - COVID-19 emergency
#PharmaTechnologyDoesNotStop - COVID-19 emergency
Following the government instructions to implement an effective containment…

Highly potent active pharmaceutical ingredients (HPAPIs) represent significant innovation for pharmaceutical companies in preparing new medicines for patients. They employ new, small molecules which are active in lower doses, with reduced side effects. A significant proportion of new active ingredients under development will be classified as highly potent, suggesting an increase in the therapeutic efficacy of these products. While most of the HPAPIs will be used as anti-cancer, others may be classified as hormones, narcotics, and retinoids.
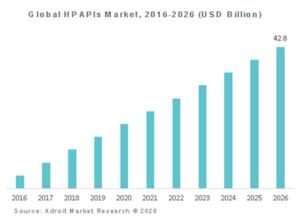
The introduction of highly potent APIs incurs new manufacturing problems and challenges. Indeed, containment of highly potent APIs in a pharmaceutical manufacturing environment has not always been compatible with efficiency. On top of that, as it involves hormones and cytostatic drugs, HPAPIs production can have carcinogenic or mutagenic effects to exposed operators. They should therefore be handled with specific precautions.
While containment technology provides necessary safety features for operators and the environment, it has historically added complexity, time, and inefficiency to manufacturing processes, and was typically batch oriented. However, recent advances in containment on pharmaceutical process equipment based on streamlined design and automation have made containment features more efficient and capable of running on continuous production lines.
Ideally, these add-ons perform their safety duties without interrupting overall production workflow. Generally, it can be assumed that high containment features added to process equipment do not improve the process in any way and, conversely, may have at least a minimally adverse effect on production. Logic dictates that creating one or more barriers or safeguards to protect operators may restrict or complicate certain equipment operations.
Of course, any potential impediments have huge upsides. The first, of course, is safety. Another is freedom of movement: these barriers usually allow the operator to work in the production room without full PPE and respirators and can help reduce other production site containment measures that might otherwise be necessary.
Let’s discuss the types of containment barriers typically utilized on two of the pharmaceutical industry’s most prominently used equipment categories: tablet presses and complementary tablet dedusters.
A drawback is that, since they are typically stainless steel, α/β split butterfly valves tend to be quite heavy and require adequate support, often in the form of a dedicated cart or support arm. To counteract this pitfall, lighter, semi-disposable versions of split butterfly valves comprised of sanitary polymer have been introduced in the last few years and are starting to be installed more frequently on process equipment. In addition to being significantly lighter, the polymer solutions eliminate the need for cleaning and are substantially less expensive.




Following a WIP cycle, the equipment still needs to be opened, wiped down with alcohol in some areas and then air dried before it can be swabbed. Clean In Place cycles have received attention in the pharma industry, but have never been truly achieved on tablet presses and dedusters due to an inherent limitation; namely, this type of process would restrict drying as the equipment would never be opened.
Amazingly, this is only a partial list of possible containment components and solutions. With so much to consider, it’s important to stay focused on an overarching principle: It’s a big game of keep away. The fewer potential touchpoints between operator and API, the safer – and, by definition, the more automated – the production process.
Fortunately, containment features on process equipment are becoming increasingly modular and are no longer mere clunky afterthoughts that get bolted on. Some of these features can be added to or upgraded on existing equipment. Other features get integrated into the design of the equipment from the beginning.
Some tablet dedusters, for example, can be supplied as standard dust tight units that can be upgraded at any point in the future to OEB 3 or even OEB5 containment levels. The fully enclosed design of the standard version keeps the API inside regardless of requirement level. This is achieved by adding pre-determined modules onto a base machine already prepared and designed with high containment in mind – such as a set of high containment inlet or outlet valves, or a WIP cart – that directs all water distribution inside the machine, including cleaning media supply, flooding and spraying, and drainage upon wash cycle completion.


Running a WIP cycle on a tablet press or deduster, however, means that some manual intervention is still needed to finish off the cleaning process. Typically, tablet presses or dedusters must be opened after a WIP cycle to be dried and swab tested. Again, the benefit of the WIP cycle is that it normally removes most of the API to which operators should not be exposed and wet any residual API to the point that it cannot become airborne. With such a WIP system, operators no longer need PPE or respirators when performing final cleaning operations. Going one step further, when using other process equipment such as fluid bed driers or pan coaters, it is possible to run a complete CIP cycle that includes drying and swabbing of product contact surface areas inside. Limiting operator exposure is not simply a question of using physical barriers between the product and the operator, it is also rethinking the process to reduce or remove operator intervention. If the equipment can be automated to run and be cleaned remotely without operator intervention in the room, then any potential for exposure is de facto reduced and the equipment can be used for continuous manufacturing.