The CUB-X is an innovative and versatile tablet inspection system, which can be used for OSD product & process development, as well as for IPC sample analysis and full batch inspection & sorting.
It is equipped with novel spectroscopy and laser technologies allowing for high-speed highly accurate non-destructive measurement of tablet thickness, API’s mass fraction and moisture content.
VERSATILE, ACCURATE AND SCALABLE
The CUB-X is an advanced, highly accurate cGMP PAT analyser designed for the real-time characterisation of solid oral dosage (OSD) forms, supporting applications from early pharmaceutical R&D through in-process control (IPC). Integrating state-of-the-art NIR-SRS spectroscopy technology, the system delivers highly accurate and precise non-destructive quantification of Critical Quality Attributes (CQAs).
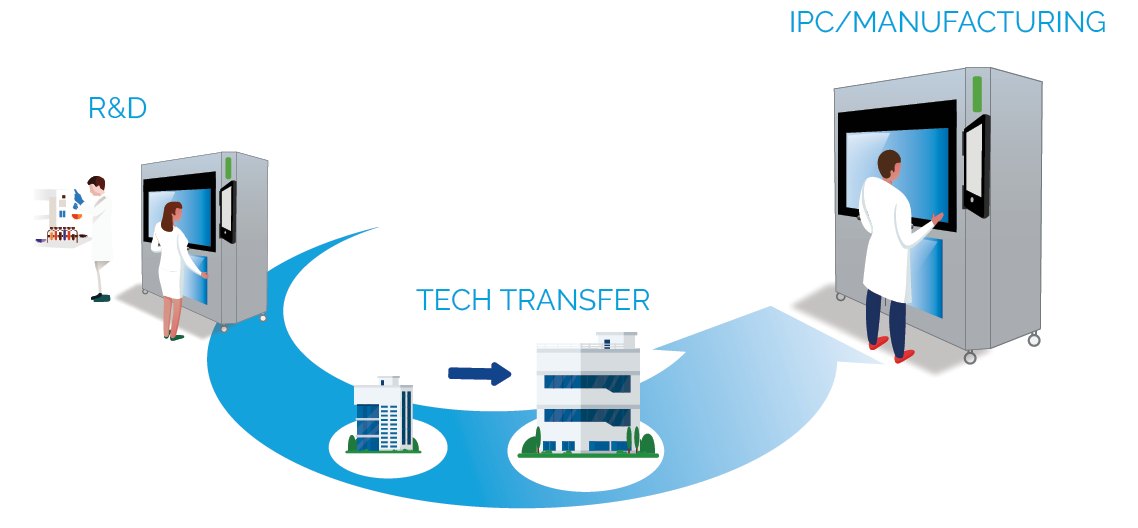
FROM R&D TO MANUFACTURING
The high-quality analytical data produced by the CUB-X support the development of robust chemometric models, enabling precise and non-destructive prediction of various CQAs such as Mass, API fraction, Assay, Content Uniformity, Hardness, Thickness, Moisture content, Porosity, and Temperature. The large quantity of data generated in R&D mode can support the formulation development, the Design of Experiment (DoE) study and the product filing to the authorities.
Engineered for exceptional operational flexibility, the analyser can process single objects in a true First-In–First-Out mode or operate with sampling rates up to 400 objects per hour, enabling seamless use across drug development and In-Process Control (IPC) testing. This allowing larger samples interpretation and higher sampling frequency. Being technology-agnostic the CUB-X analyser can be easily installed in any manufacturing line around the world.
AUTOMATIC, EASIER, MORE PRECISE AND MORE ROBUST PAT
PAT technologies for tablet are perceived sophisticated by many, hence companies are reluctant to implement them. The CUB-X has been designed to reshape the use of PAT in the OSD product development and manufacturing, the software is automatically guiding the user step by step into the different phases of the entire workflow: from setting up DoE studies, configuring the equipment, and tagging samples, to data acquisition, ordination, storage, and chemometric model application, all the way through to day-to-day routine operation and little specialized expertise is required to keep it runnig.
EMBEDDED TECHNOLOGIES
A laser line-scanning sensor profiles each tablet, while passing under the sensor, measuring its thickness with a precision of 50 µm. The tablet laser profiler also verifies the correct position and orientation of the tablet on the conveyor belt before it passes under the NIR-SRS sensor.
Each tablet is inspected by the multipoint NIR-SRS (Near-Infra-Red Spatially Resolved Spectroscopy) probe at a high frame rate, allowing multiple spectral acquisitions per tablet. An accurate prediction of one or multiple ingredients’ mass fraction – including water – is then done by chemometric modelling. The SRS technology allows to cover a larger tablet surface and a deeper measurement penetration compared to single-point NIR reflection techniques, resulting in more accurate prediction of the CQA’s.
Samples can be sent to an embedded 4-parameter tablet tester at a rate of up to 400 tablets/h, measuring tablet mass, thickness, diameter/length, and hardness. The high sampling rate is achieved thanks to a purpose-designed sampling gate, which feeds tablets one by one directly to the measurement instruments
| Maximum inspection speed | 20.000 tablets/h |
| Maximum sampling rate | 400 tablets/h |
| Minimum product width | 4 mm |
| Maximum product width | 24 mm |
| Minimum product height | 3 mm |
| Maximum product height | 10 mm |
| Thickness measurement accuracy | ± 0,05 mm |
| Mass accuracy | ± 1 mg in stable environment |
| Hardness measurement accuracy | ± 2 N (4-400 N range) |
| Length measurement accuracy | ± 0,1 mm |
| Air extraction | Integrated Hepa filter H13 |
| Requested compressed air | Min 500 L/min - Min. 6 bar |
| Nosie level | ≤70 dB (A) |
| Power supply | 380 V 3ph + N + PE |
| Frequency | 50 Hz / 60 Hz |
| Polymer contact parts | FDA approved |
